Core Concepts – Thermochemistry Vocabulary
In this article you’ll learn the definitions and gain a better understanding of the main vocabulary terms for thermochemistry such as the different kinds of systems and what it means to have surroundings. The terms are listed in alphabetical order for your convenience!
Topics Covered in Other Articles
What is Thermochemistry?
Thermochemistry is the study of heat changes in chemical reactions and phase changes (such as boiling or freezing). In thermochemistry, heat is a form of energy, and not just the temperature that something is. Scientists study thermochemistry in order to determine how much energy (or how much of a product) can be created or will be consumed. For instance, the burning of fuel, production of fireworks, and powerplants all use thermochemistry!
Vocabulary and Definitions
- Endothermic – If a reaction is endothermic, it absorbs energy. Think of it like you have to put energy EN-to and ENdothermic process.
- Exothermic – If a reaction is exothermic, it releases energy. Think of it like if you have an exothermic process, the energy will EXit.
- Heat – in thermochemistry heat is defined as form of energy. It’s the transferal of energy from one piece of matter to another due to temperature difference and is normally represented by the letter “q”. An important note is that naturally, heat is transferred from a “hotter” object to the lower temperature object.
- Law of Conservation of Energy – states that energy cannot be created or destroyed in any chemical/physical process.
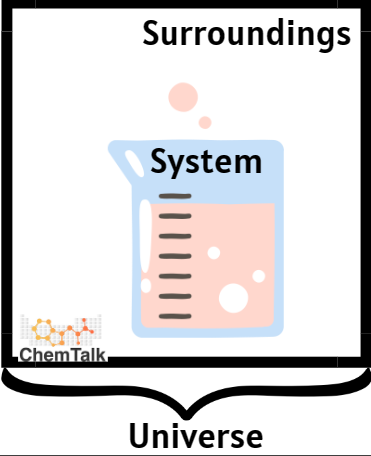
- Surroundings – in thermochemistry the surroundings are everything else in the universe that isn’t in the chemical equation.
- System – a system is the entire reaction that you’re looking at.
- Closed system – this is a system where matter (or the contents of the reaction) cannot leave, but energy can. For example, a covered pot on the stove is a closed system because whatever you’re cooking isn’t able to leave but the steam (or the energy) is able to escape.
- Isolated system – this is a system where neither matter nor energy are able to escape. If you have an insulated cooler, this acts as an isolated system where your food cannot escape and the energy (the stuff that keeps your food cold) also cannot escape.
- Open system – this is a system where matter and energy can escape. If you have a pot on the stove with no lid, then the contents can escape and so can the energy.

Make sure to check out the other related articles for more topics related to thermochemistry!