Introduction:
The layout of the periodic table and where an element is located, can tell you a lot about its properties! In this article, you’ll learn how you can figure out what an element’s charge is by looking at where it’s placement on the periodic table! Here you’ll find a link to a downloadable periodic table with charges.
What is a Charge?
A charge on an atom comes from the difference between protons (positive charge) and electrons (negative charge) present in the atom. Each element has a unique number of protons in the center of its atom, or its nucleus. However, atoms can often lose or gain electrons so it can be tricky to determine their charge. If for instance, one fluorine atom (9 protons) has 10 electrons giving it a -1 charge, but another fluorine atom has 9 electrons giving it a neutral charge, how do we know which charge to assume fluorine has??
Trends in Charges

These trends are handy to memorize! Below you’ll find a full periodic table with the most common charges of every element!
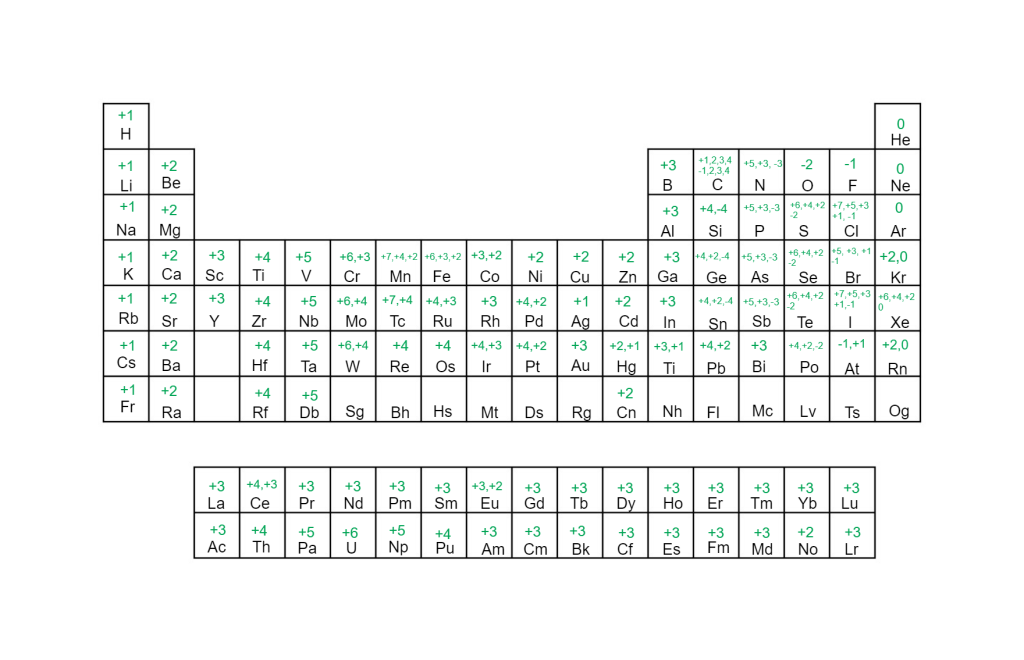
More Ways to Find a Charge
Now let’s say you don’t have your handy periodic table with charges with you! There are multiple ways to find an elements charge.
- Subtract the number of protons from the number of electron
- Ex. An atom of iron (Fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. That means that the charge of iron is 26-23= +3.
- You can also find the charge of an element by balancing a compound. For more information on how to do this click here.
For More Help, Watch Our Interactive Video Explaining Charges and Ions!
Related To The Periodic Table:
- What is Atomic Number?
- Periodic Table with Names
- What are Valence Electrons?
- The Periodic Table Explained
- Trends in the Periodic Table
Be sure to check out our INTERACTIVE PERIODIC TABLE to learn more about each element!

