What is Ionization Energy?
Ionization energy, also called ionization potential, is a property that all elements on the periodic table have. So what is the definition of ionization energy? It is the amount of energy required to remove an electron from a neutral atom, which forms an ion. It is usually measured in kJ/mol, and the measurement is based on an isolated atom in its gaseous phase. Let’s learn how to calculate it, what is meant by first and second ionization energy, and how it trends on the periodic table.
Ionization energy can be shown by the equation:
X + first ionization energy → X+ + e–
Where
- X is neutral atom
- X+ is an ion of atom X with a single positive charge
- e– is an electron with a single negative charge
What is meant by “first” ionization energy?
In the equation, the “first ionization energy” refers to the ionization energy required to remove a neutral atom’s first electron, giving an ion with a single positive charge. Second ionization energy is the amount of energy required to remove a second electron from a 1+ ion (meaning an ion with a single positive charge), giving an ion with a 2+ charge.
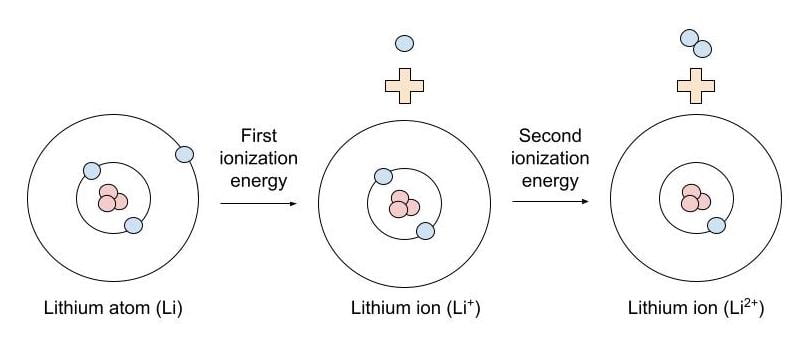
What is an ion?
An ion is a positively or negatively charged atom—it gets the charge by having a number of electrons unequal to that of its protons. For example, the sodium ion, also written as Na+, has 11 protons and 10 electrons. There is one more proton than there are electrons, making the ion positively charged. The number of protons for any atom or ion is always constant (the number of protons determines the atomic number).
How to Calculate Ionization Energy
Ionization potential for hydrogen can be calculated using the following equation:
E = hcRH(1/n2), where
- E is energy of the electron (or the amount of energy it takes to remove the electron, ionization energy)
- h is Planck’s constant = 6.626 * 10-34 Js (joules*seconds)
- c is the speed of light = 3.00 * 108 m/s (meters/second)
- RH is Rydberg constant = 1.097 * 107 m-1 (1/meters)
- n is the principal quantum number (or energy level) of the electron
After plugging in the values of constants, the equation becomes:
E = (2.18 * 10-18 J)(1/n2)
From here, you can plug in the value of the energy level of the electron to find the amount of energy needed to remove it.
Ionization Energy Trend on the Periodic Table
For the ionization energy trend on the periodic table, we will assume that we are always referring to elements’ first ionization energy. In general, (first) ionization energies increase toward the top right corner of the periodic table, with helium having the highest ionization energy. Before we break down the trend into its period and group trends, let’s talk about a major contributing factor to this trend: the octet rule.
The Octet Rule
According to the octet rule, atoms strive to have a complete set of 8 valence electrons. This is because this configuration provides the most stability for the atom. Group 18 noble gases have an octet of electrons, which causes them to be chemically inert and nonreactive. Noble gas atoms do not react with other elements because they are already extremely stable, due to the octet of electrons they have.
How does the octet rule relate to the trend of ionization energies on the periodic table? Since atoms strive to have an octet, each atom’s ionization energy differs based on how many electrons they have. Let’s see this relationship directly with the ionization energy period trend.
Period Trend
Across a period, ionization energies increase. As mentioned before, elements strive to have complete octets of valence electrons. As elements have successively more electrons across a period, atoms get closer and closer to their goal. So, removing an electron becomes harder and harder, and the ionization energy increases, as atoms approach an octet. It is very easy to remove an electron from an atom that is very far from an octet.
A group 1 element that has one valence electron will readily lose its electron in order to have an octet of electrons. Thus, group 1 elements have very low ionization energies. It takes very little energy to remove an electron because the atom can be more stable without it.
On the opposite end of the spectrum, group 17 elements have very high ionization energies. This is because, with 7 valence electrons, halogens want to gain one more electron to form an octet. Losing an electron puts them farther away from their goal, and thus, it takes much more energy to remove an electron.
Group Trend
Down a group, ionization energies decrease. This is because as you go down a group, electrons are located in successively higher energy levels, farther away from the attraction of the nucleus. Furthermore, down a group, there are more electrons between the outside valence electrons and the nucleus. These middle electrons help “shield” the outer electrons from the attractive forces of the nucleus. Therefore, it is easier to remove an electron from lower in a group.
The Periodic Table Trend on a Graph
As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization energies. Between groups 1 and 18, ionization potentials generally increase across a period.

