Core Concepts
In this tutorial, you will learn about exergonic and endergonic reactions and how they describe what happens to energy during a reaction.
Topics Covered in Other Articles
- What is Gibbs Free Energy?
- Endothermic vs Exothermic Reactions
- Types of Chemical Reactions
- What is Entropy?
- Cell Metabolism: The Energetic Machinery of Life
What is an Exergonic Reaction?
In an exergonic reaction, free energy is released. Due to the energy being released, these reactions do not need outside factors to proceed, and we also refer to them as favorable or spontaneous. This is because the final state is lower in Gibbs free energy than the starting reactant. It is important to remember, that even though a reaction is spontaneous does not mean it will happen quickly. Exergonic reactions have a negative change in Gibbs free energy because energy is being released.
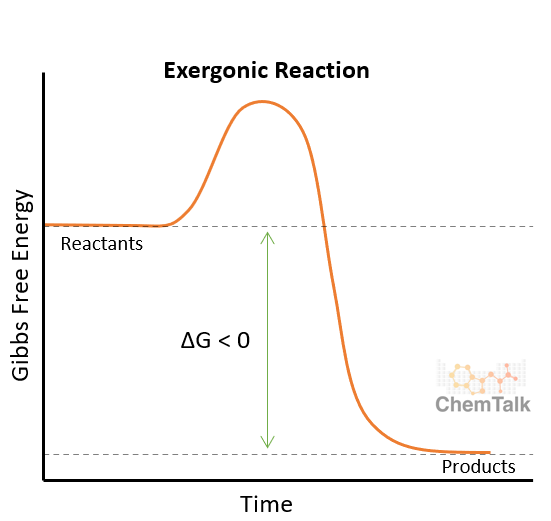
The graph above shows that the difference between the reactants and the peak of the reaction is smaller than the difference between the peak and the products. This indicates that the reaction favors the products over the reactants in an exergonic reaction. Another way to look at this is that the products are further down than the reactants on the y-scale.
Exergonic reactions occur all the time, even inside your own body! Glycolysis is a great example of an exergonic reaction because it is where a molecule of glucose spits into two molecules of pyruvate. As our bodies break down the molecule of glucose, it releases energy that can be used. Mixing sodium and chlorine to create sodium chloride (table salt) represents another example of an exergonic reaction because the process releases energy. (Watch the reaction in this video!)
What is an Endergonic Reaction?
Endergonic reactions are the opposite of exergonic reactions. This means that in an endergonic reaction, the reaction absorbs free energy. Because there is more energy at the end of the reaction than there is at the beginning of it, the reaction requires energy to be supplied in order for it to proceed. This is why endergonic reactions are referred to as unfavorable or unspontaneous. Some ways we can assist endergonic reactions is by adding heat to the reaction or by coupling it to an exergonic reaction. Endergonic reactions have a positive change in Gibbs free energy because energy is being absorbed.
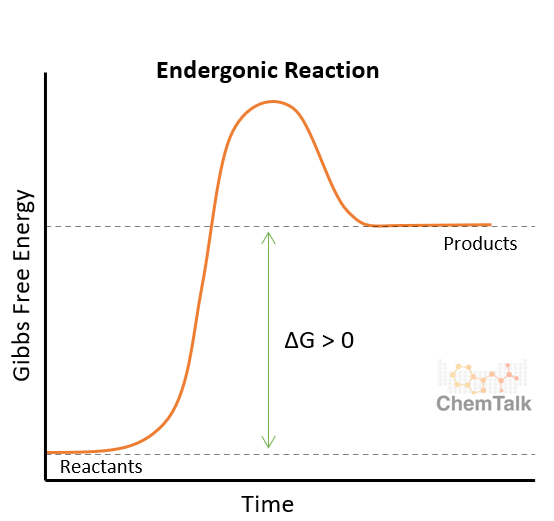
In the graph above, the difference between the reactants and the peak of the reaction is larger than the difference between the peak and the products. This shows that the reactants are favored over the products in an endergonic reaction.
Similarly to exergonic reactions, endergonic reactions occur all the time. Plants are consistently taking in energy from the sun and using it to convert carbon dioxide and water into sugar and oxygen through the process of photosynthesis. Another simple example of an exergonic reaction is melting. When ice melts into water, it uses heat energy from its surroundings.
Spontaneity
We talked about how exergonic reactions are spontaneous and endergonic reactions are unspontaneous. This is because exergonic reactions do not require outside energy to occur, while endergonic reactions do. Spontaneity can often be confused with how fast a reaction occurs, but we are referring to how a reaction favors the formation of the products versus the reactants. Exergonic reactions favor the products over the reactants; therefore, they are spontaneous, and vice versa for endergonic reactions. For example, the oxidation of iron (formation of rust) is an exergonic reaction even though it is very slow.
Exothermic and Endothermic?
Exothermic and endothermic reactions are often confused with exergonic and endergonic reactions. The reason that they sound similar and can often be confused is that they describe exergonic and endergonic reactions when the energy released or absorbed is heat energy. An exothermic reaction results in an increase in the temperature of the surroundings. In parallel, an endothermic reaction results in a decrease in the temperature of the surroundings.
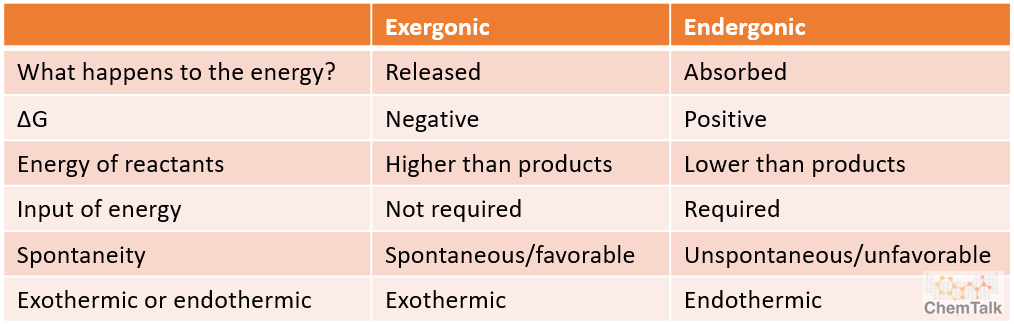
Exergonic vs Endergonic Overview
Practice Problems
Problem 1: Cellular respiration is a metabolic pathway that breaks down glucose to produce ATP. What type of reaction is this?
Problem 2: What does it mean when the ΔG of a reaction is equal to zero?
Problem 3: If the energy of the reactants is less than the energy of the products, what type of reaction is it?
Solutions
1: It is an exergonic reaction.
2: This means that the chemical reaction is in equilibrium. When this happens, the relative concentration of products and reactants does not change. This happens because the products and reactants are converting into each other at an equal rate.
3: It is an endergonic reaction.

