Core Concepts
In this article, you will learn about the effects of intermolecular forces (IMFs). You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension.
Topics Covered in Other Articles
- Intermolecular forces
- Properties of Solids, Liquids, and Gases
- The melting and boiling point of water
- Surface Tension and Vapor Pressure
- States of Matter
- Osmotic Pressure
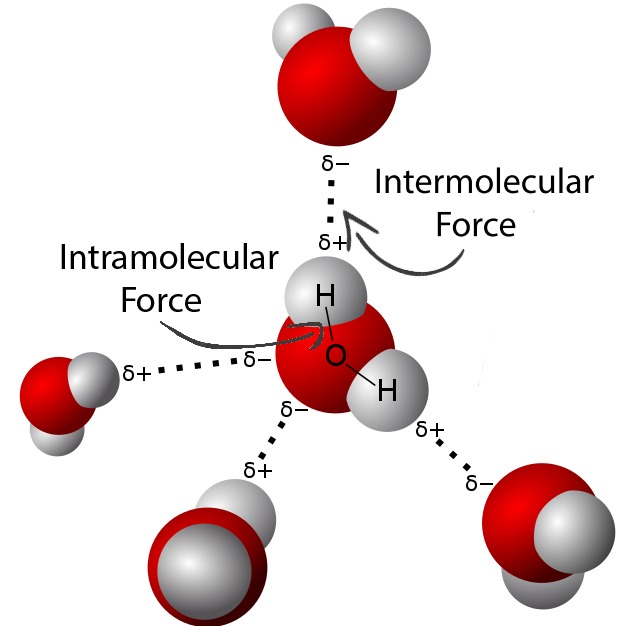
What are Intermolecular Forces?
Intermolecular forces (IMFs), as its name would suggest, are forces between molecules. They are electrostatic interactions between charged molecules. Though IMFs include both attractive and repulsive interactions, the term is usually only used for attractive interactions between molecules (having strong IMFs between two molecules means the force of attraction between the two is strong).
There are different types of intermolecular forces: London dispersion forces, dipole-dipole forces, ion-dipole forces, and hydrogen bonding. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc.
How Intermolecular Forces Influence Physical Properties
Boiling/Melting points
The boiling point is the temperature at which a liquid can turn into a gas, and the melting point is the temperature at which a solid can turn into a liquid.
Having strong intermolecular forces means that the attraction between the molecules is strong. Therefore, if a substance has stronger IMFs, it is harder to pull its molecules away from each other, resulting in the substance having a higher boiling point and melting point.
For example, propane (C3H8), has a boiling point of -42°C while methane (CH4), has a boiling point of -161.6°C. Propane has such a higher boiling point than methane because it has stronger London dispersion forces (larger molecule) and stronger IMFs overall than methane.
Viscosity
Another effect of intermolecular forces is viscosity. Viscosity is the measure of a fluid’s resistance to flow. A high viscosity results in a thicker and stickier fluid. Viscosity is a result of intermolecular forces. Stronger intermolecular forces cause a fluid to have a stronger attraction to itself, giving it a high viscosity.
Glycerol, for example, has much stronger intermolecular forces than water because it contains 3 -OH hydroxyl groups capable of hydrogen bonding, while water only has one. This is why water flows very well while glycerol doesn’t.

Vapor Pressure and Volatility
The strength of the intermolecular forces of a liquid affects the liquid’s vapor pressure. Vapor pressure is the pressure exerted by vapor on the surface of a liquid. An increase in volatility, which is the tendency of a substance to evaporate, results in an increase in vapor pressure. Stronger intermolecular forces give a liquid a stronger attraction to itself. This makes it harder for the liquid to evaporate, resulting in lower volatility and lower vapor pressure.
Surface Tension
One of the effects of intermolecular forces is surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. The stronger the intermolecular forces, the greater the surface tension.

Solubility
Another one of the effects of intermolecular forces is solubility. The stronger the intermolecular forces between the solute and solvent, the greater the solubility. The phrase “like dissolves like” helps predict the solubility of a solute in a solvent. Polar molecules dissolve polar molecules through dipole-dipole forces, and nonpolar molecules dissolve nonpolar molecules through London dispersion forces. Polar molecules and nonpolar molecules don’t dissolve well with each other.
Influence of Intermolecular Forces Practice Problems
Order the following compounds from lowest boiling point to highest:
- Helium gas He
- Isobutyl alcohol C4H10O
- Acetone (CH3)2CO
- Water H2O
Influence of Intermolecular Forces Solutions
Solution: Helium gas, Acetone, Water, Isobutyl alcohol
Explanation: Since having stronger intermolecular forces increases boiling point, this problem is to rank the compounds from weakest to strongest intermolecular forces. Helium Gas is the smallest molecule here and is only capable of London dispersion forces, so it has the lowest boiling point. Acetone is capable of dipole-dipole forces but not hydrogen bonding, which water and Isobutyl alcohol are capable of, so acetone has the second lowest boiling point. Water and Isobutyl alcohol both have one -OH hydroxyl group, but Isobutyl alcohol is larger and has stronger London dispersion forces, so Isobutyl alcohol has the highest boiling point.