Core Concepts
In this organic chemistry tutorial, you will learn the basics of the aldol condensation reaction, its mechanisms, the enol intermediate, and the Claisen Condensation.
Topics Covered in Other Articles
- Condensation Reactions
- Carbonyl Functional Group
- Nucleophiles
- Electrophiles
- Properties of Acids and Bases
- Cycloaddition Reactions
What is the Aldol Condensation?
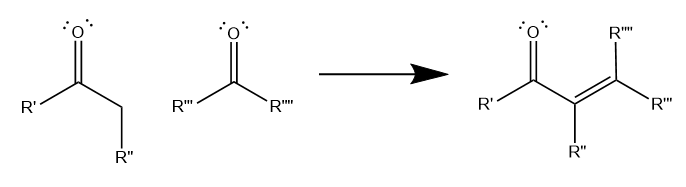
The aldol condensation involves the reaction of two carbonyl-containing molecules to form an unsaturated carbonyl molecule, with a C=C double bond between two carbons adjacent to the carbonyl, as shown below. Generally, the aldol condensation must involve at least one ketone, while the other molecule can be either a ketone or an aldehyde as starting material. The structure of the unsaturated carbonyl product depends on the structure of the reactant carbonyls.

Though aldol condensations can occur with a variety of R groups, the most common form of the reaction involves a methyl ketone and an aldehyde, which form an unbranching unsaturated carbonyl.

Additionally, all aldol condensations result in a net loss of two hydrogens and one oxygen. Essentially, this net loss is equivalent to one water molecule, which is why chemists call this reaction a “condensation.”
The Enol Intermediate
The enol molecule is an important intermediate in aldol condensations and shows up in many other organic reactions. By definition, the structure involves a C=C double bond (-ene-) and an adjacent alcohol (-ol), forming what chemists call an enol (-ene- plus -ol).

Ultimately, this combination of functional groups tends to be unstable, due to the favorability of carbonyl groups relative to enol groups. Consequently, enol molecules quickly undergo keto-enol tautomerization under basic or neutral conditions, forming what chemists call an enolate ion, containing a carbonyl and a carbanion. Alternatively, acidic conditions stabilize the enol molecule somewhat, but enol molecules tend to be particularly reactive.

Essential Aldol Condensation Mechanisms
Interestingly, aldol condensations can occur in either acidic or basic conditions, but the reaction mechanisms under either condition are different. In either condition, both mechanisms involve the deprotonation of the carbon next to the carbonyl. This is because the electron-withdrawing effect of the carbonyl increases the acidity of the adjacent carbon’s hydrogens. Note that both mechanisms involve the molecules HA and B, which indicate a generic acid and generic base, respectively.
Aldol Condensation under Basic Conditions
To begin the condensation reaction, a base deprotonates the carbon next to the carbonyl group on the first molecule, forming an enolate molecule. This creates a nucleophilic carbon, due to the newly freed electron pair as a result of the deprotonation.

Second, the nucleophilic carbon attacks the second molecule at the carbon double bonded to oxygen, which is electrophilic due to the oxygen’s electronegativity. This pushes an electron pair from the carbonyl double bond onto the oxygen, forming an anion. However, this oxygen is quickly protonated, resulting in an alcohol.

Third, an elimination reaction occurs where the acidic carbon is deprotonated a second time, forming another carbanion intermediate. The free electron pair proceeds to form a double bond with the carbon carrying the alcohol, ejecting the alcohol. Notably, chemists call this step an E1cB reaction, characterized by a strong acid deprotonation (the central carbon) and a relatively poor leaving group (the alcohol). The “E1″ means that it is a unimolecular elimination, while the “cB” means “conjugate base,” which relates to the carbanion intermediate.

Finally, the unsaturated carbonyl product is formed.
Aldol Condensation under Acidic Conditions
To begin, the carbonyl of the first molecule is protonated while the adjacent carbon is deprotonated, forming an enol molecule.

Second, one electron pair from the double bond then attacks the carbonyl of the second molecule. This forms an oxygen ion which is quickly protonated, similar to the mechanism under basic conditions. Additionally, this nucleophilic attack allows the reformation of the carbonyl on the first molecule.

Third, an elimination reaction occurs. Unlike under basic conditions, this step proceeds predominantly through an E1 reaction pathway (although little amounts of E2 reactions might be present), which involves a strong leaving group and carbocation formation. To begin, the newly formed alcohol is protonated a second time, creating a strong leaving group of a water molecule. As the water molecule leaves, a carbocation forms, which makes the hydrogens of the central carbon more acidic. This allows for a second deprotonation, freeing another pair of electrons that form a double bond.

Claisen Condensations
One important aldol condensation example is the Claisen Condensation. Specifically, this reaction involves ester molecules and follows the base condition mechanism. However, a carboxy salt with the same alkyl group as the esters must serve as the base. This is to prevent the replacement of the carboxy group of the carbonyl molecules. Another important distinction is that the end product is a dicarbonyl molecule, rather than an unsaturated monocarbonyl.

To begin a Claisen Condensation, the carbon next to the first molecule’s carbonyl is deprotonated. The resulting carbanion then attacks the electrophilic carbonyl carbon of the second molecule. Consequently, the C=O bond reforms, ejecting the carboxy group.

Next, if the Claisen Condensation takes place in water, then an alcohol group replaces the remaining ester group. This results in a carboxylic acid group.

However, this carboxylic acid molecule lacks stability and will break down at high temperatures. Specifically, a decarboxylation occurs, involving a circular movement of electrons that cleaves the carboxylic acid, releasing carbon dioxide. The resulting enol molecule then tautomerizes into a ketone. Decarboxylation occurs in many important biological pathways, most notably in the citric acid cycle.